Despite owning the same genetic material, our cells carry out highly specialized functions and vary from each other in many phenotypic aspects. Interestingly, when in troubled times (i.e., under stressful conditions) certain cells change their characteristic protein expression and turn into something completely unexpected, to help us survive. Fasolino and group shed light on such an instance where pancreatic ductal cells express immune surface proteins that potentially help protect against T cell infiltration in Type 1 Diabetes.
Type 1 diabetes (T1D), also called juvenile diabetes is an autoimmune disease where beta cells of the pancreas are destroyed by self-reactive T cells. The loss of beta cells abrogates insulin production which makes patients suffering from T1D completely dependent on pharmaceutical insulin for the rest of their lives. Not only is that a temporary solution, but it also deteriorates one’s quality of life. Hence, attempts in understanding the mechanism of disease elaborately and the search for better therapies is ongoing. Among these gaps in knowledge remains an unanswered question- What triggers T cells to travel to the pancreas and carry out this selective elimination of beta cells?
To explore the particular functions of cells in pancreatic microenvironment during T1D, Fasolino and colleagues carried out a large-scale single-cell analysis of human pancreatic tissues. Single-cell transcriptome analysis involves breaking down the tissue into cells followed by sequencing of individual cells’ mRNA. For such analysis, a massive amount of data is generated, since gene expression of each cell is obtained. This data is analyzed by using computational methods and provides unprecedented insights into the tissue’s microenvironment. The pancreatic tissue samples used in this study were donated by patients suffering from T1D and also patients who had autoantibodies against beta cells but where T1D has not manifested yet. Tissue from people with no T1D was used as a control. In this blog article, we are focusing on one of the many results of this study.
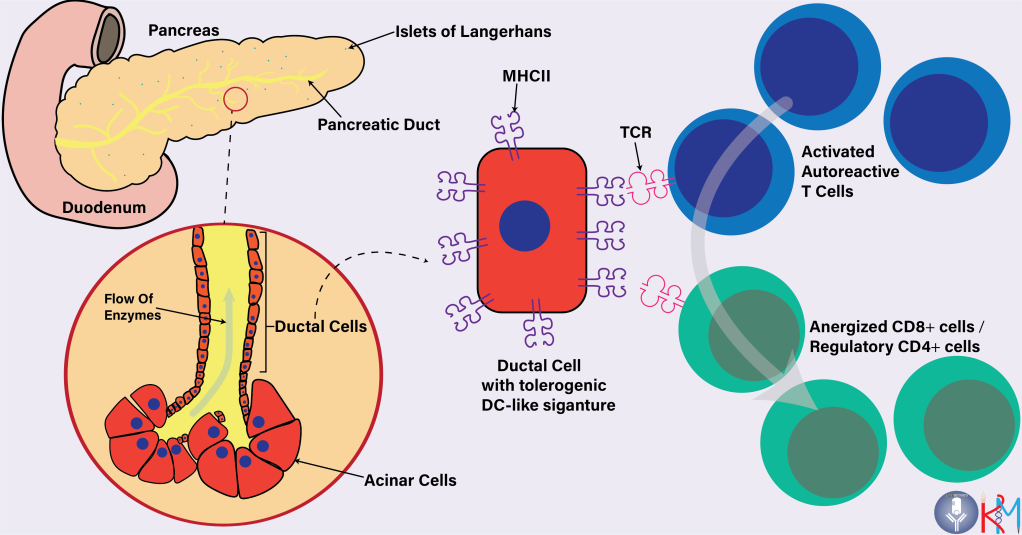
The researchers categorized the ~80,000 cells obtained from single cell sequencing of the donated tissues into endocrine (alpha, beta, delta & epsilon), pancreatic polypeptide, stellate, endothelial, acinar, ductal, and immune cells. Although a small organ, the pancreas does have a very complex cellular architecture. The Islets of Langerhans make up the endocrine and pancreatic polypeptide cells, while the pancreatic duct consists of ductal cells (producing bicarbonate to neutralize stomach acid) surrounded by enzyme-secreting acinar cells at their ends. Immune cells like resident macrophages are present throughout the pancreas, while T cells and DCs can be found during T1D.
In this study, to the surprise of the researchers, genes associated with antigen presentation (like MHCII) and interferon pathways were highly enriched in T1D affected ductal cells as compared to that in controls. MHCII is a surface protein expressed by antigen-presenting cells, especially Dendritic Cells (DCs). Despite T1D and controls’ pancreas carrying a similar number of cells, 91% of T1D ductal cells had MHCII enriched in them, as compared to merely 31% in controls.
To explore this further, the gene expression of these ductal cells was compared to that of DCs. They found that the genes enriched in the ductal cells were significantly similar to the gene expression of a subset of DCs, called tolerogenic DCs. As the name suggests, tolerogenic DCs play a regulatory role in the reduction of T cell activity, thus suppressing immune response instead of inciting an immune response (like the DCs we often read about). The ductal cells did not express costimulatory molecules like CD80/86 and upregulated VSIR surface protein, both of which help in curtailing T cell response.
This study hence reveals a previously unknown function of ductal cells in T1D whereby adapting a tolerogenic DC-like phenotype, ductal cells potentially help in reducing the autoimmune T cells from damaging the beta cells.
Because mRNA is not a direct indicator of cellular phenotype, inferences obtained through single-cell transcriptomics often need further validation. To this end, Fasolino and the group carried out traditional and imaging mass cytometry, both of which identify and quantify proteins. They also stained and imaged control and T1D pancreatic sections with markers for ductal cells and MHCII. Through all these methods, they confirmed that ductal cells indeed had higher expression of MHCII during T1D. Moreover, they also observed T helper cells and myeloid cells surrounding these DC-like ductal cells in the diseased pancreas.
The regulatory immune gene signatures expressed by ductal cells showed how the exocrine region helps protect the endocrine region from autoimmune attacks. Although the eventual destruction of the beta cells does signify that it’s not enough, a better understanding of the mechanism and fortification of the same could open new avenues of treatment. Due to the nature of this study, functional experimentation and validation are still lacking, but we are sure scientists are already working on it!
Source:

Article author: Kevin Merchant. Kevin is a MS student at LMU Munich, Germany, who is passionate about Immunology and writing. He aims to simplify latest research so that it becomes accessible to all.
Editor: Sutonuka Bhar. Sutonuka is a PhD candidate at the University of Florida. Her work focuses on host immune responses against viruses and bacterial membrane vesicles.
Check out Antibuddies’ blog post “New Role for Ductal Cells in Type I Diabetes”.
Tweet


Leave a comment