This generation has experienced how a novel pathogen can cause a pandemic leading to turmoil, destruction of life, and deterioration of living conditions. What about pathogens that have been in our environments for decades? Malaria, spread by mosquito bites is prevalent throughout the equatorial regions (Sub Saharan Africa, Asia, and Latin America) and also occurs in temperate zones at lower rates. Unfortunately, we have seen that global warming can shift mosquito habitats by facilitating warm and humid conditions, risking its spread and infection worldwide. Currently, about 229 million cases of Malaria occur every year, leading to ~400,000 deaths worldwide. Symptoms of Malaria include high fever, headaches, intense chills, and in serious cases, jaundice, anemia and even death.
Let us dive into the modus operandi of Malaria and how a vaccine development strategy from Tripathi and group from John Hopkins University can help to curb it.
Parasites of the Plasmodium genus cause malaria- Plasmodium falciparum being the deadliest one. These single celled parasitic eukaryotes (much more complex than viruses and bacteria) depend on other living organisms for nutrition and reproduction. Female Anopheles mosquitoes and vertebrates are the main hosts for Plasmodium. Try visualizing the Plasmodium life cycle:
We start in the salivary glands of female mosquitos where highly motile form of these parasites, called Sporozoites, reside. When these mosquitos bite humans (their favorite food being our blood) they inject their saliva into our bloodstream. As soon as Sporozoites enter our bloodstream, they swim to the liver and infiltrate liver cells to reproduce. In the liver they morphos into Merozoites and multiply asexually. Thousands of Merozoites reenter the bloodstream to invade our RBCs (Red Blood Cells). As expected, here they multiply again, ruthlessly bursting open the RBCs as they progress; soon lowering the host’s RBC count drastically (anemia). Some of these Merozoites convert to male or female gametocytes and keep circulating in the blood. When another mosquito feasts on an infected host’s blood, these gametocytes are also taken up. This initiates the final steps of Plasmodium life cycle the fusion of the male and female gametocytes in the mosquito midgut. The fusion produces an egg (oocyte) that goes through many steps of development, eventually giving rise to Sporozoites that will travel to the mosquito salivary glands. In this way, the Plasmodium life cycle ends (or restarts).
Usually, vaccine development focuses on human stages of Plasmodium. Considering their life cycle, another way of curbing spread could be attacking the parasites residing in the mosquitoes. Such vaccines are referred to as Transmission Blocking Vaccines (TBV). By vaccinating people using TBVs, their bodies can produce antibodies specific to gametocyte and subsequent mosquito stage antigens. So, when a mosquito sucks blood from these vaccinated humans, it takes up antibodies too, which will then hamper the Plasmodium life cycle from progressing. To this end, Tripathi and colleagues set out to find antigens that would help in TBV development.
Their first step was to design P. falciparum antigens which will be most effective as vaccines. These researchers carried out mRNA expression studies in gametocytes of P. falciparum, filtering genes which expressed either in high levels or expressed on the gametocyte cell surface (easy access). They chose 16 genes, from the sequences of which they produced purified recombinant proteins. These proteins were then formulated into vaccines and administered to mice. These proteins – acting as antigens – induce production of antibodies specifically against them. The efficiency of the antibodies was tested by feeding the blood of vaccinated mice to mosquitoes infected with P. falciparum and checking for marked reduction in Plasmodium oocyte numbers. Through this complex process, they found that antibodies produced by 2 of the 16 genes, Pf77 and PfMDV-1, showed ~85% oocyte density reduction. This reduction significantly reduced transmission and made these 2 protein antigens the best candidates for future research.
But at what stages are these proteins present? Surprisingly, aside from having high expression at Plasmodium gametocyte stage, Pf77 and PfMDV-1 also had detectable expression in all other developmental stages (like Sporozoites and Merozoites). Additionally, these proteins had very little sequence variation across different strains of P. falciparum found around the world and largely similar proteins were expressed by other Plasmodium species too. These data prove that the selected antigens are widely expressed at various stages and are universal within species, ergo can easily be targeted, making them excellent candidates for vaccine.
Lastly, they also saw that Ghanaian adults who previously suffered from P. falciparum infection had antibodies against these antigens- proving that these proteins are readily immunogenic (our bodies can efficiently produce antibodies against them.)
Till now, introducing TBV vaccines to the market has been difficult, since many limitations often pop up in pre-clinical and clinical trials. Although these two antigens give us hope, there are many basic science ‘knowledge gaps’ that need to be clarified before they can be tested preclinically. These include, but are not limited to, better structural understanding of antibody-antigen binding, protein folding & conformations, and adjuvants/conjugates screening. But with two promising candidates identified, humankind have already taken a step in the right direction.

Article author: Kevin Merchant. Kevin is a MS student at LMU Munich, Germany, who is passionate about Immunology and writing. He aims to simplify latest research so that it becomes accessible to all.
Editor: Sutonuka Bhar. Sutonuka is a PhD candidate at the University of Florida. Her work focuses on host immune responses against viruses and bacterial membrane vesicles.
Check out Antibuddies’ blog post ” Stopping Malaria at its Source”.
Tweet


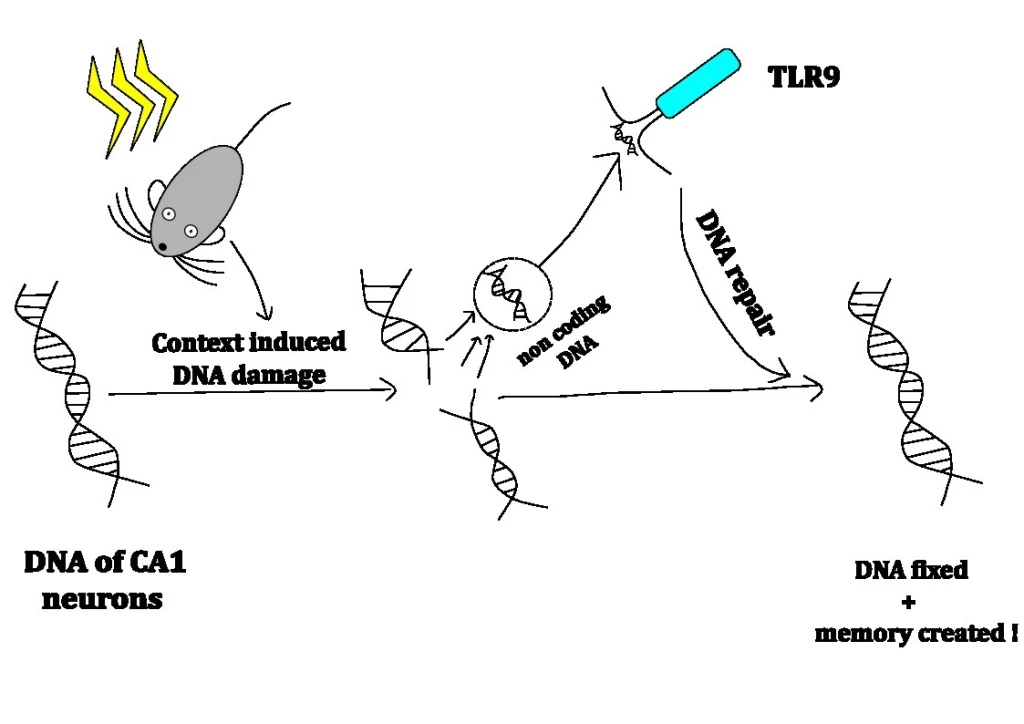
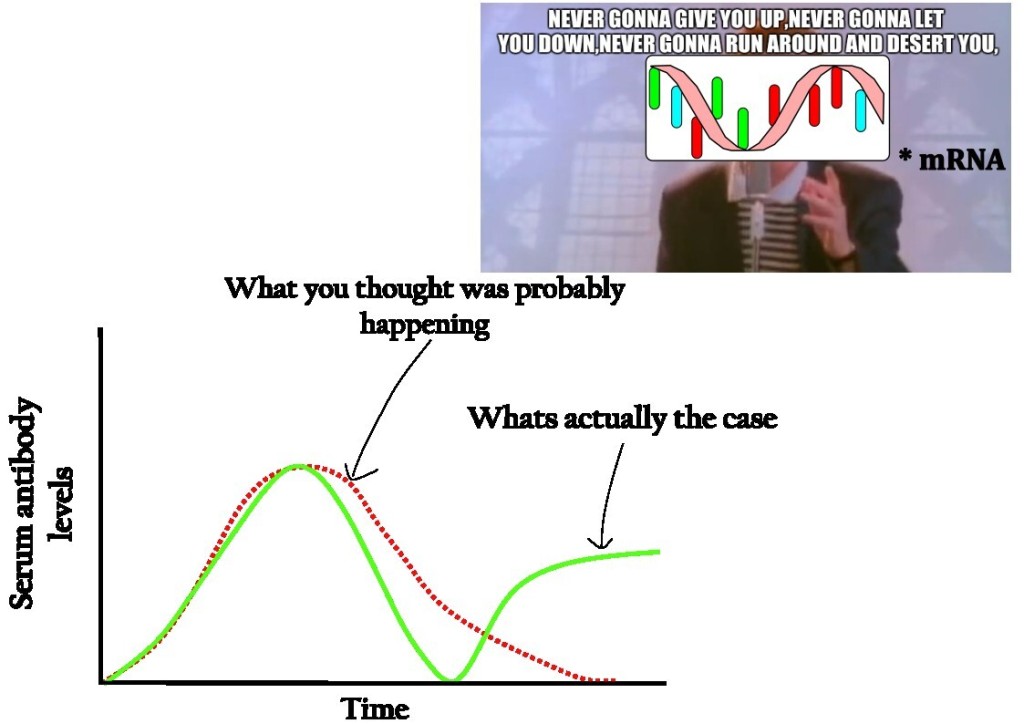
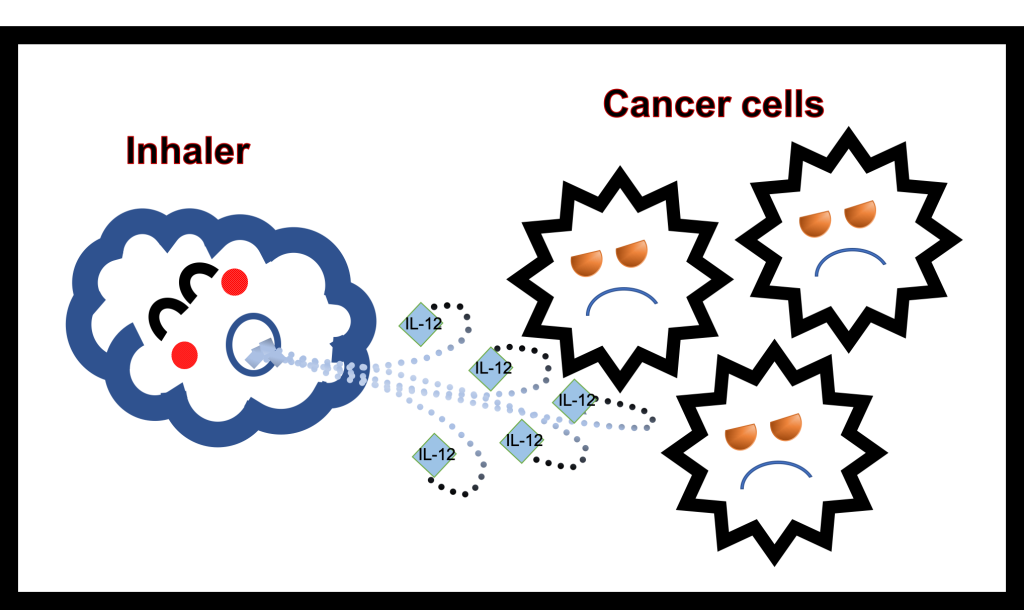

Leave a comment